A Vaccine Primer Toolkit
Learn About The Safety of Vaccines
Table of Contents
GHW’s Vaccine Primer
01. WHY VACCINES?
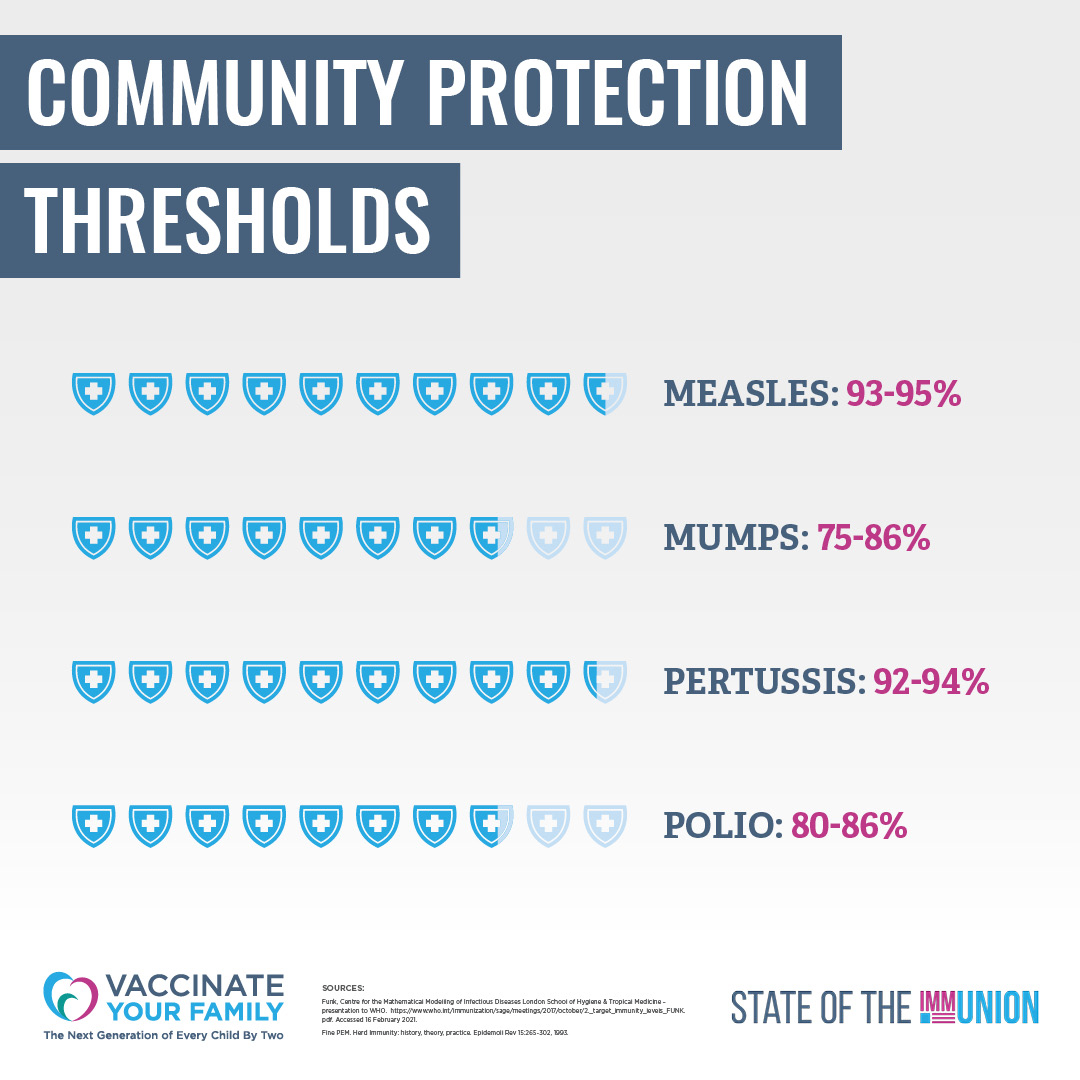
02. HOW DO VACCINES WORK?
03. HOW ARE VACCINES MADE?
04. HOW ARE VACCINES TESTED?
05. WHAT HAPPENS AFTER FDA APPROVAL?
06. Are Vaccines Monitored?
07. WHO IS RECOMMENDED TO RECEIVE VACCINES?
08. HOW DO PEOPLE PAY FOR VACCINES?
09. IF YOU HAVE A REACTION TO A VACCINE?
10. ADDITIONAL VACCINE RESOURCES
Overview
Increase Vaccination Rates
The CDC’s Bridge Access Program stands as a pivotal initiative in the nationwide effort to combat the COVID-19 pandemic by ensuring equitable access to vaccines. Established to provide free COVID-19 vaccines to eligible individuals, this program serves as a critical bridge for those without health insurance or with insufficient coverage. By eliminating financial barriers, the Bridge Access Program aims to bolster vaccination rates and curb the spread of the virus.
FAQs
Cover. Address. Benefit
FAQs:
1. What is the CDC’s Bridge Access Program? The Bridge Access Program is an initiative by the CDC aimed at offering no-cost COVID-19 vaccines to adults lacking health insurance or possessing insurance that doesn’t cover all vaccination expenses.
2. Why is the Bridge Access Program important? This program addresses disparities in vaccine access by providing a solution for those who may face financial obstacles in obtaining COVID-19 vaccines. By ensuring free access, it contributes to broader vaccination coverage and, consequently, public health safety.
3. Who can benefit from the Bridge Access Program? Eligible beneficiaries include uninsured adults and individuals with health insurance that fails to cover the full cost of COVID-19 vaccines. This encompasses a significant portion of the population vulnerable to vaccine accessibility issues.
03. HOW ARE VACCINES MADE?
The process of each vaccine
Coverage:
The Bridge Access Program caters to a diverse demographic, extending its reach to those who fall through the gaps of conventional healthcare coverage. This includes:
- Uninsured Adults: Individuals lacking health insurance coverage.
- Underinsured Adults: Those whose insurance policies inadequately cover COVID-19 vaccine expenses.
04. HOW ARE VACCINES TESTED?
Safety and Effectiveness
05. WHAT HAPPENS AFTER FDA APPROVAL?
Additional Hurdles
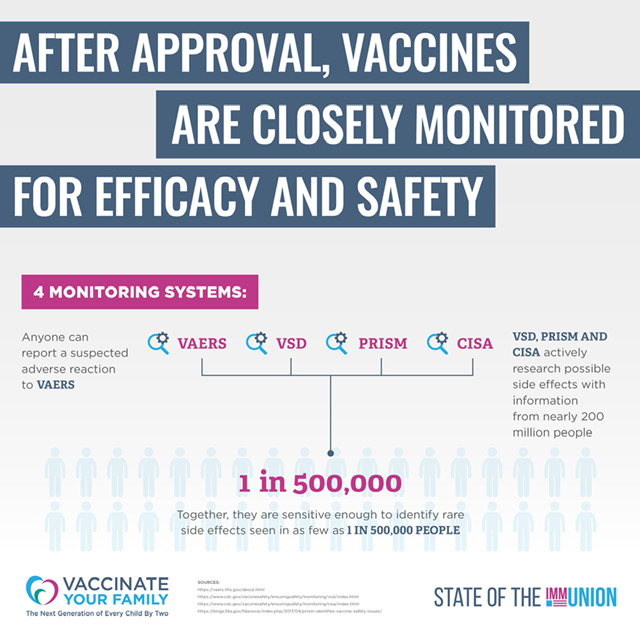
06. Are Vaccines Monitored?
Safety & Effectiveness After Approval
There are several systems in place to continue monitoring vaccines for their safety and effectiveness after they are approved by the FDA. Three of the key systems are:
- VAERS, or the Vaccine Adverse Event Reporting System, collects adverse event reports, but it relies on individuals to report injuries and is therefore not meant to form a
comprehensive database of possible vaccine side effects. - VSD, or the Vaccine Safety Datalink, has been a continuing collaboration since 1990 between the CDC’s Immunization Safety Office and eight health care organizations across the country. This network conducts studies based on questions or concerns raised from the medical literature and reports to VAERS. In addition, when new vaccines are recommended, or if changes are made in how a vaccine is recommended, VSD will monitor the safety of these vaccines and recommendations.
- CISA, or the Clinical Immunization Safety Assessment Project, began in 2001 to better understand individual risk for adverse events following immunization. CISA addresses vaccine safety issues, conducts high quality clinical research and assesses complex clinical adverse events following vaccination.
07. WHO IS RECOMMENDED TO RECEIVE VACCINES?
Vaccines aren’t just for children anymore
Vaccines aren’t just for children anymore. Children of all ages, including preteens and teens, as well as adults and pregnant people are recommended for certain vaccines.
- Children & Teens: Children can be protected from more infectious diseases than ever before. You can look at the CDC’s recommended vaccination schedules to learn more about the vaccines infants, children and teenagers should receive.
- Adults: Adults may need to receive vaccines depending on their age, whether they have any other health conditions such as asthma or heart disease, and what they do for a living. The CDC has an online quiz to help adults learn which vaccines they may need.
- Pregnant People: All pregnant people should receive a Tdap (tetanus-diphtheria-pertussis) vaccine during every pregnancy as well as a flu vaccine if they are pregnant during the Fall and Winter months. These vaccines not only protect pregnant people, but the baby when it’s born thanks to antibodies from their mothers. You can learn more about these vaccines and their safety on our partner’s, Vaccinate Your Family’s, website.
To learn more about all of the diseases that vaccines can prevent, check out Vaccinate Your Family’s website.
08. HOW DO PEOPLE PAY FOR VACCINES?
Insured or Not Insured
People in the U.S. either have private health insurance, public health insurance (meaning Medicare, Medicaid or, in the case of children, CHIP), or are not insured at all. While there are some safety nets in place to guarantee access to vaccines, they still have many holes.
- Private Health Insurance: The Affordable Care Act required all private health insurance plans to cover vaccines at no charge to patients, including those for both children as well as adults. However, some plans were “grandfathered,” meaning employers only had to cover vaccines when they started a new insurance plan, and some have yet to begin a new plan. This means some people with private health insurance still have to pay a copay or other fee to receive vaccines for themselves, their spouses or their children.
- Medicare: Medicare covers flu and pneumococcal vaccines at no cost to patients. Any other adult vaccine may or may not be covered depending on whether the Medicare recipient has a Part D plan and on how that plan covers vaccines.
- Medicaid: We do not yet have universal coverage for vaccines under Medicaid. Each state’s plans are different and may or may not cover vaccines for adults and for pregnant people at no cost.
- CHIP: Each state has a CHIP, or Children’s Health Insurance Plan. CHIP provides health coverage to children whose families meet certain income requirements. Those requirements differ by state. Not all children who are covered by CHIP have coverage for vaccines.
- Vaccines for Children: Luckily, there is a program called Vaccines for Children, or VFC. VFC was created by Congress in the 1990s to ensure no child was denied a vaccine because their families couldn’t afford it. As a result, vaccination rates among children have risen to an all time high.
09. IF YOU HAVE A REACTION TO A VACCINE?
Vaccine Injury
Since the program’s inception over 30 years ago, it has paid over $4 billion in compensation and legal fees. That means about one in every 1 million people vaccinated has been compensated.
The program is not funded by taxpayers, but by an excise tax on each vaccine administered. It also does not prevent someone from filing a civil lawsuit. If a person’s case is not decided within 240 days of filing, or if they do not like the ruling of this special court, they can choose to move their claim to civil court.
10. ADDITIONAL VACCINE RESOURCES
Toolkits and Other Educational Resources
• The CDC has developed A Guide for Community Partners which includes Best Practices for CBOs/FBOs. •Resources for Faith-based and Community Organizations Fighting COVID-19
highlights a variety of ways that FBOs and CBOs can access federal funding or otherwise engage in partnerships with government.
•The Ad Council has developed community-specific toolkits for Black, Hispanic/Latino, faith, public health, and employer communities with messaging tips, FAQs and other resources to help organizations increase confidence in COVID-19 vaccines. Included within these resources are videos, study guides, event templates and more.
• Questions & Answers About COVID-19 Vaccines | The Ad Council (getvaccineanswers.org) • Toolkit for Black Communities | Ad Council COVID-19 Initiative (blackcommunityvaccinetoolkit.org) • Toolkit for Faith Communities | Ad Council COVID-19 Initiative (faithcommunityvaccinetoolkit.org) • Toolkit for Hispanic Communities | Ad Council COVID-19 Initiative
(hispaniccommunityvaccinetoolkit.org)
• Groups such as Choose Health Life and Urban Strategies have developed educational resources.
Would you like to join the COVID-19 Community Corps? As a member, you’ll receive timely, accurate information to share with your family, friends, and neighbors. By encouraging them to get vaccinated, you’ll help protect them – and allow all of us to safely gather together again.
As a Corps member, you’ll get resources to help you build vaccine confidence in your community, including:
• Fact sheets on vaccine safety, tips on how to talk with friends and family about the importance of vaccination, and hints for planning and attending community events
• Social media content to share with your followers
• Regular email updates with the latest vaccine news and resources to share
You can sign up here: https://wecandothis.hhs.gov/covidcommunitycorps. We hope you will join.
As stated by the CDC, “Health equity is when all members of society enjoy a fair and just opportunity to be healthy as possible.” COVID-19 vaccinations present an important opportunity to ensure that all members of society can be as healthy as possible.
• Racial Equity Guidebook developed on behalf of Black Coalition against COVID-19 addresses equitable vaccine use in the community, including through faith-based and community partnerships.
• Made to Save is national grassroots effort to ensure communities hardest hit by the pandemic have equitable access to the COVID-19 vaccines and accurate, timely information. Check out how to partner with them and join their training on 4/27 at 7pm ET – “Talking to Friends and Family about the COVID-19 Vaccines” – RSVP
• Faith4Vaccines is an inclusive, multifaith movement comprised of local and national religious leaders, as well as medical professionals, who are working together to identify and resolve current gaps in vaccine mobilization, outreach, and uptake.
• FEMA document on protecting civil rights during disasters, including during COVID-19: https://www.fema.gov/sites/default/files/documents/fema_civil-rights_bulletin_03-04-2021.pdf
•If your community is interested in supporting state and local vaccine programs, use the CDC’s locator to determine local vaccine efforts, contact your state or local health department or state Association of Immunization Manager (AIM) to find out when, where, and how vaccines are being made available in your community. Also, check in with your local Community Health Center, which may have received funding to increase vaccine access.
• You can also contact pharmacies like those within Walmart and Rite Aid sites who presented on the webinar. You can also find pharmacies participating in the Federal Retail Pharmacy program here.
• More information about State health officials can be found at the Association of State and Territorial Health Officials (ASTHO).
• More information about Local health officials can be found at National Association of County and City Health Officials (NACCHO).
• Learn more about Community Health Centers at the National Association of Community Health Centers.
• The Association of Immunization Managers (AIM) released a two-page overview How Can Faith Leaders Help End the COVID-19 Pandemic? Support the COVID-19 vaccination effort.
• The National Forum on COVID-19 Vaccine has a variety of materials and resources supporting equitable vaccine engagement and community-based partnerships, including information on:
• Equitable Vaccine Implementation,
• Engaging Community-Based Organizations to be Vaccination Partners, and
• Increasing Vaccine Confidence through Communication and Community Engagement.
•FEMA has also developed a guidebook communities can consider in setting up vaccination centers of all sizes. – Community Vaccination Centers Playbook | FEMA.gov.
• Health Equity Task Force – The Office of Minority Health (hhs.gov)
• Coronavirus Disease 2019 (COVID-19) | CDC
• Coronavirus (COVID-19) Response | FEMA.gov
• Partner Coordination Efforts to Strengthen Infection Prevention and Control Practices •Public Health and Faith Community Partnerships: Model Practices to Increase Influenza Prevention Among Hard-to-Reach Populations
• Mobilizing for Action through Planning and Partnerships (MAPP)
• Engaging with Community- and Faith-Based Organizations in Public Health Emergencies
GHW’s acknowledges All In: Partnering with Faith and Community in COVID-19 Vaccination Efforts for their additional vaccine resources
GLOSSARY
VACCINE ALPHABET SOUP: A GLOSSARY
Choose a Location
● FDA: Food and Drug Association
● WHO: World Health Organization
● ACIP: Advisory Committee on Immunization Practices
● AAP: American Academy of Pediatrics
● AAFP: American Academy of Family Physicians
● ACP: American College of Physicians
● AMA: American Medical Association
● ACOG: American College of Obstetricians and Gynecologists
● MMWR: Morbidity and Mortality Weekly Report
● VAERS: Vaccine Adverse Event Reporting System
● VSD: Vaccine Safety Datalink
● CISA: Clinical Immunization Safety Assessment Project
● VFC: Vaccines for Children program
● CHIP: Children’s Health Insurance Plan
● VICP: Vaccine Injury Compensation Program